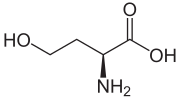
Homoserine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(S)-2-Amino-4-hydroxybutanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.538 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H9NO3 | |
| Molar mass | 119.12 g/mol |
| Melting point | 203 °C (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Homoserine (also called isothreonine) is an α-amino acid with the chemical formula HO2CCH(NH2)CH2CH2OH. L-Homoserine is not one of the common amino acids encoded by DNA. It differs from the proteinogenic amino acid serine by insertion of an additional −CH2− unit into the sidechain. Homoserine, or its lactone, is the product of a cyanogen bromide cleavage of a peptide by degradation of methionine. Homoserine is an intermediate in the biosynthesis of three essential amino acids: methionine, threonine (an isomer of homoserine), and isoleucine.[1]
Applications
[edit]Commercially, homoserine can serve as precursor to the synthesis of isobutanol and 1,4-butanediol.[2] Purified homoserine is used in enzyme structural studies.[3] Also, homoserine has played important roles in studies to elucidate peptide synthesis and synthesis of proteoglycan glycopeptides.[4] Bacterial cell lines can make copious amounts of this amino acid.[5][2]
Biosynthesis
[edit]Its complete biosynthetic pathway includes glycolysis, the tricarboxylic acid (TCA) or citric acid cycle (Krebs cycle), and the aspartate metabolic pathway.[clarification needed] It forms by two reductions of aspartic acid via the intermediacy of aspartate semialdehyde.[6] Specifically, the enzyme homoserine dehydrogenase, in association with NADPH, catalyzes a reversible reaction that interconverts L-aspartate-4-semialdehyde to L-homoserine. Homoserine kinase and homoserine O-succinyltransferase convert homoserine to phosphohomoserine and O-succinyl homoserine, respectively.[5] Homoserine is produced from aspartate via the intermediate aspartate-4-semialdehyde, which is produced from β-phosphoaspartate. By the action of homoserine dehydrogenases, the semialdehyde is converted to homoserine.[7]

Other biochemical roles
[edit]L-Homoserine is substrate for homoserine kinase, yielding phosphohomoserine (homoserine-phosphate), which is converted by threonine synthase to L-threonine.
Homoserine is converted to O-succinyl homoserine by homoserine O-succinyltransferase. O-succinyl homoserine is a precursor to L-methionine.[8]
Homoserine inhibits aspartate kinase and glutamate dehydrogenase.[5] Glutamate dehydrogenase reversibly converts glutamate to α-ketoglutarate and α-ketoglutarate coverts to oxaloacetate through the citric cycle. Threonine acts as another allosteric inhibitor of aspartate kinase and homoserine dehydrogenase, but it is a competitive inhibitor of homoserine kinase.[8]
References
[edit]- ^ Tanaka M, Kishi T, Kinoshita S (September 1961). "Studies on the Synthesis of l -Amino Acids: Part III. A Synthesis of l -Homoserine from l -Aspartic Acid". Agricultural and Biological Chemistry. 25 (9): 678–679. doi:10.1080/00021369.1961.10857862. ISSN 0002-1369.
- ^ a b Huang JF, Zhang B, Shen ZY, Liu ZQ, Zheng YG (July 2018). "Metabolic engineering of E. coli for the production of O-succinyl-l-homoserine with high yield". 3 Biotech. 8 (7): 310. doi:10.1007/s13205-018-1332-x. PMC 6037649. PMID 30002999.
- ^ Akai S, Ikushiro H, Sawai T, Yano T, Kamiya N, Miyahara I (February 2019). "The crystal structure of homoserine dehydrogenase complexed with l-homoserine and NADPH in a closed form". Journal of Biochemistry. 165 (2): 185–195. doi:10.1093/jb/mvy094. PMID 30423116.
- ^ Yang W, Ramadan S, Yang B, Yoshida K, Huang X (December 2016). "Homoserine as an Aspartic Acid Precursor for Synthesis of Proteoglycan Glycopeptide Containing Aspartic Acid and a Sulfated Glycan Chain". The Journal of Organic Chemistry. 81 (23): 12052–12059. doi:10.1021/acs.joc.6b02441. PMC 5215661. PMID 27809505.
- ^ a b c Liu P, Zhang B, Yao ZH, Liu ZQ, Zheng YG (October 2020). Zhou NY (ed.). "Multiplex Design of the Metabolic Network for Production of l-Homoserine in Escherichia coli". Applied and Environmental Microbiology. 86 (20). Bibcode:2020ApEnM..86E1477L. doi:10.1128/AEM.01477-20. PMC 7531971. PMID 32801175.
- ^ Berg, J. M.; Stryer, L. et al. (2002), Biochemistry. W.H. Freeman. ISBN 0-7167-4684-0
- ^ Viola, Ronald E. (2001). "The Central Enzymes of the Aspartate Family of Amino Acid Biosynthesis". Accounts of Chemical Research. 34 (5): 339–349. doi:10.1021/ar000057q. PMID 11352712.
- ^ a b Petit C, Kim Y, Lee SK, Brown J, Larsen E, Ronning DR, et al. (January 2018). "Reduction of Feedback Inhibition in Homoserine Kinase (ThrB) of Corynebacterium glutamicum Enhances l-Threonine Biosynthesis". ACS Omega. 3 (1): 1178–1186. doi:10.1021/acsomega.7b01597. PMC 6045374. PMID 30023797.
